What is Endoscope Certification: Check into These Products
Aug 13, 2024
In the world of medical technology, endoscopes play a crucial role in various diagnostic and therapeutic procedures. Their certification is essential for ensuring that these devices meet high standards of quality and performance. This blog explores the certification details of different types of endoscopes and highlights why Mole Medical stands out as a premier supplier.

What is Endoscope Certification
The Role of CE Certification
CE (Conformité Européenne) certification indicates that an endoscope meets the health, safety, and environmental protection standards required in the European Economic Area (EEA). This mark is mandatory for products sold within the EU and signifies that the device complies with European Union directives. For endoscopes, CE certification ensures that they have undergone rigorous testing to verify their safety and effectiveness in medical procedures.
CE certification covers various aspects, including device performance, quality control, and clinical efficacy. It means that the endoscope has been evaluated by a notified body, which confirms that it meets all necessary regulations and standards. For medical professionals, this certification provides assurance of the product’s reliability and suitability for use in clinical settings.
The Importance of FDA Approval
FDA (Food and Drug Administration) approval is crucial for endoscopes marketed in the United States. This certification demonstrates that the device has been thoroughly evaluated for safety, effectiveness, and quality. The FDA requires extensive documentation and testing before granting approval, which includes clinical trials, manufacturing process validation, and performance assessments.
FDA approval signifies that the endoscope has met the rigorous standards set by U.S. regulatory authorities. This certification helps ensure that medical professionals can trust the device for accurate diagnostics and effective treatment. It also provides a level of credibility and reliability that is essential for maintaining high standards in patient care.
Navigating KGMP Certification
KGMP (Korea Good Manufacturing Practice) certification is a standard required for medical devices in South Korea. It ensures that the manufacturing process adheres to strict quality control measures, guaranteeing the safety and efficacy of the endoscope. KGMP certification is crucial for products distributed in the South Korean market and reflects the device’s compliance with local regulations.
This certification covers various aspects of the manufacturing process, including quality management systems, production controls, and documentation practices. For endoscope manufacturers, obtaining KGMP certification is a testament to their commitment to maintaining high-quality standards and ensuring that their products meet local regulatory requirements.
NMPA Certification and Its Significance
NMPA (National Medical Products Administration) certification is required for medical devices sold in China. This certification ensures that the endoscope meets Chinese safety and quality standards. The NMPA, formerly known as CFDA (China Food and Drug Administration), oversees the approval process for medical devices and requires comprehensive testing and documentation.
NMPA certification provides assurance that the endoscope has undergone rigorous evaluations to meet the specific requirements of the Chinese market. This includes clinical trials, safety assessments, and performance testing. For international manufacturers, NMPA certification is essential for entering the Chinese market and expanding their global reach.
ISO13485 and ISO9001 Certifications
ISO13485 is an international standard specifically for medical device quality management systems. It outlines the requirements for a comprehensive quality management system that ensures the design, development, production, and post-market activities of medical devices, including endoscopes, meet high standards of safety and effectiveness.
ISO9001, on the other hand, is a broader quality management standard applicable to various industries, including medical devices. It focuses on overall quality management principles and emphasizes continuous improvement, customer satisfaction, and consistent product quality.
Both ISO13485 and ISO9001 certifications are crucial for endoscope manufacturers. They provide a framework for maintaining high-quality standards and ensuring that products consistently meet regulatory requirements and customer expectations.
Key Considerations When Choosing Endoscope Certification Products
Assessing Certification Relevance
When selecting endoscope certification products, it is important to consider the relevance of the certifications to your specific market and regulatory environment. Different certifications may be required depending on the region where the device will be used. For example, CE certification is essential for products sold in Europe, while FDA approval is necessary for the U.S. market.
Understanding the certifications relevant to your region helps ensure that the endoscope meets all necessary regulatory requirements. This not only facilitates compliance but also provides confidence in the device’s safety and performance. It is advisable to choose products that have the necessary certifications for your specific market to avoid regulatory hurdles and ensure smooth usage.
Evaluating Manufacturer Credentials
The manufacturer’s credentials and experience play a significant role in the quality of endoscope certification products. Reputable manufacturers with a track record of adhering to regulatory standards and maintaining high-quality practices are more likely to produce reliable and effective devices.
When choosing endoscope certification products, consider manufacturers who have obtained multiple certifications, such as CE, FDA, and ISO13485. This indicates their commitment to meeting international standards and their capability to produce high-quality devices. Additionally, manufacturers with a strong reputation in the industry are more likely to provide excellent customer support and reliable products.
Checking for Comprehensive Testing and Quality Assurance
Comprehensive testing and quality assurance are crucial for ensuring the performance and safety of endoscope certification products. Devices should undergo rigorous testing to verify their functionality, durability, and accuracy. This includes performance testing, safety assessments, and clinical evaluations.
Endoscopes that have passed comprehensive testing and quality assurance processes provide greater confidence in their reliability. Look for products with detailed documentation of their testing procedures and quality control measures. This information can be obtained from the manufacturer or supplier and provides insights into the device’s performance and adherence to regulatory standards.
Endoscope Certification Product 1: Disposable Video Laryngoscope For Adults
Certification and Compliance
The Disposable Video Laryngoscope for adults boasts several key certifications, including CE, FDA, NMPA, and ISO13485. These certifications confirm that the device adheres to stringent safety, efficacy, and quality standards across different markets. CE (Conformité Européenne) ensures compliance with European safety standards, FDA (Food and Drug Administration) approval denotes adherence to U.S. regulatory standards, NMPA (National Medical Products Administration) certification aligns with Chinese regulations, and ISO13485 guarantees that the manufacturing processes meet international standards for medical devices.
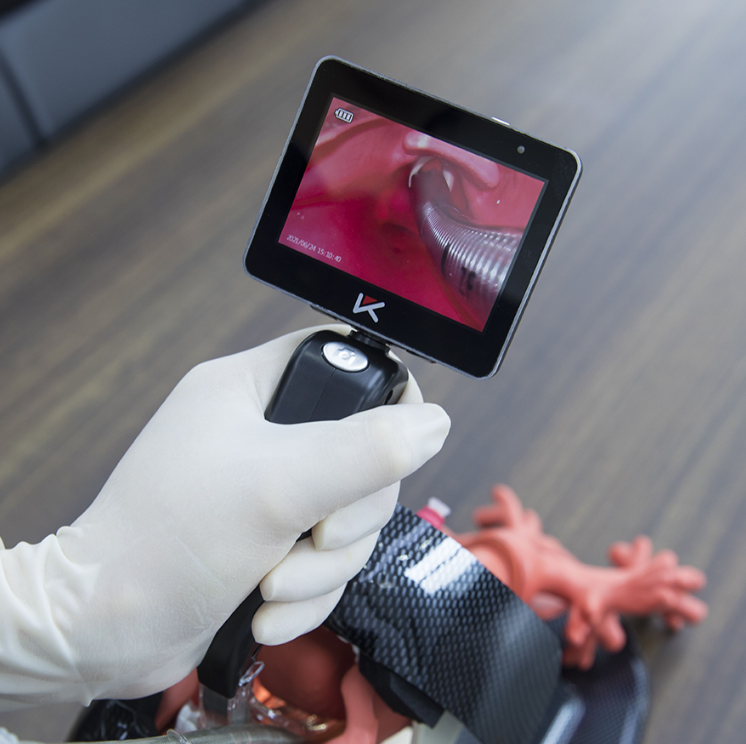
High-Resolution Video Technology
One of the standout features of this laryngoscope is its high-resolution video technology. The device is equipped with a superior camera that provides clear, real-time visuals of the larynx and vocal cords. This advanced imaging capability enhances intubation accuracy, allowing medical professionals to perform procedures with greater precision. The high-resolution visuals are crucial for both routine and complex intubations, ensuring that clinicians can see detailed structures and make informed decisions during procedures.
Ergonomic and Durable Design
The design of the Disposable Video Laryngoscope emphasizes both durability and user comfort. Its ergonomic handle provides a secure grip, which is essential for precise maneuvers during intubation. The device is built to withstand the rigors of clinical use while ensuring that it remains comfortable for extended periods. Additionally, the laryngoscope is portable and lightweight, making it suitable for various settings, from emergency rooms to operating theaters.
Advanced Features and Functionality
The device includes several advanced features designed to enhance its performance. An integrated high-intensity LED light system offers excellent illumination of the airway, crucial for accurate placement. The lens is treated with an anti-fog coating to maintain visibility in challenging conditions. The laryngoscope also comes with interchangeable blades of different sizes, catering to diverse anatomical requirements. Furthermore, its long battery life ensures reliable performance during prolonged procedures or emergencies, and the high-definition LCD display screen aids in quick and accurate intubation.
Endoscope Certification Product 2: Flexible Video Bronchoscope
Versatile Functionality and Application
The Flexible Video Bronchoscope is a multifunctional device designed to handle a variety of medical procedures. It supports airway examinations, bronchoalveolar lavage, tissue biopsy, and treatment of pneumothorax, among other applications. This versatility makes it an invaluable tool in managing challenging intubations and performing bronchial therapies, including targeted radiotherapy and chemotherapy. Its design also facilitates the extraction of foreign objects from the trachea and stent placement, expanding its utility across different clinical scenarios.

Robust and Durable Design
The bronchoscope features a robust electronic camera system equipped with LED lighting and CMOS technology for high-quality image processing. This modern system enhances durability and has a longer lifespan compared to conventional scopes. The operating handle connects to the display via an aviation-grade connection, ensuring a stable and secure attachment. A 40-degree twist locks the interface in place, while the insertion component’s 240° rotation capability allows for multi-directional viewing without needing to rotate the entire apparatus.
Economic and Practical Benefits
From an economic perspective, this bronchoscope offers significant value. The device’s multiple working channel sizes cater to different procedural needs, making it a cost-effective choice for medical facilities. Its high-resolution imaging system ensures clear visuals, contributing to improved diagnostic accuracy and procedural efficiency.
Clinical Applications
This flexible bronchoscope finds application in a range of clinical settings, including Intensive Care Units (ICUs), Neonatal Intensive Care Units (NICUs), respiratory medicine, anesthesiology, and emergency departments. Its versatility allows it to be used across multiple medical departments, including emergency, anesthesiology, respiratory care, otolaryngology (ENT), pediatrics, and in operating rooms and ambulances.
Endoscope Certification Product 3: Disposable Flexible Ureteroscope
Advanced Imaging and Processing Capabilities
The Disposable Flexible Ureteroscope features an HD image processing system that uses touch-type technology for intelligent operation and high-definition imaging. This advanced system incorporates sophisticated processing algorithms to enhance image quality, including contrast adjustment, noise reduction, and edge enhancement. The result is clearer and more defined visuals, which are crucial for accurate diagnosis and treatment.

Superior Maneuverability
This ureteroscope offers exceptional maneuverability with a bending angle of 275° up and down, and a small bending radius. This flexibility is vital for navigating complex urinary tract pathways, allowing for precise positioning in challenging locations. The superior maneuverability enhances the scope’s ability to visualize and access the upper urinary tract, including the kidneys, making it particularly effective for stone management and other intricate procedures.
Wide Field of View
The ureteroscope provides a 120-degree field of view, offering a comprehensive visualization of the urinary tract. This wide observation range improves diagnostic accuracy by allowing clinicians to see more area in a single image. The extensive view also aids in better detection of pathologies, leading to more efficient and effective procedures.
Why Choose Mole Medical?
Certification and Quality Assurance
Mole Medical stands out as a premier supplier of endoscopic devices due to its comprehensive range of professional certifications, including CE, FDA, KGMP, NMPA, ISO13485, and ISO9001. These certifications validate the quality and safety of Mole Medical’s products, ensuring they meet the highest standards across various markets.
Customized Solutions and Innovation
Mole Medical offers customized airway management solutions tailored to the specific needs of healthcare providers. Their strong R&D capabilities allow them to provide innovative and effective solutions for various medical procedures. The company supports OEM/ODM customization, giving clients the flexibility to adapt products to their unique requirements.
Reliable and Cutting-Edge Products
Choosing Mole Medical means investing in reliable and cutting-edge endoscopic devices. The company’s products are designed to enhance procedural accuracy, improve patient outcomes, and ensure durability and ease of use. With a commitment to innovation and quality, Mole Medical continues to be a trusted partner in the field of medical technology.

Conclusion
In summary, endoscope certification is crucial for ensuring the safety and effectiveness of medical devices. Whether it’s the Disposable Video Laryngoscope, Flexible Video Bronchoscope, or Disposable Flexible Ureteroscope, each device’s certification and advanced features play a significant role in improving clinical outcomes. Mole Medical’s commitment to quality and customization makes it an excellent choice for healthcare providers seeking reliable and innovative endoscopic solutions.
Categories
Latest Articles
How Fibre Optic Laryngoscopes Improve ENT Procedures
In modern ENT procedures, precision and visibility are key. That’s where the laryngoscope fibre optic technology comes in. Unlike traditional tools, these advanced devices use fibre optics to provide a clear, well-lit view of the throat and vocal cords. This means doctors can see more and do more—with less risk to the patient. But how ... Read more
Flexible Laryngoscopy: A Clearer Voice for Quicker Diagnoses
Flexible Laryngoscopy is a powerful tool that helps ENT specialists do just that. It uses a thin, flexible scope to view the throat, vocal cords, and airway in real-time. The procedure is quick, non-surgical, and performed right in the clinic. For patients with voice changes, chronic cough, or throat discomfort, this method offers fast answers ... Read more
Smart Solutions in Ureteroscopy: Mole Medical’s Disposable Flexible Scope vs Karl Storz Legacy
In the field of urology, the name “ureteroscope Karl Storz” has long stood for precision and reliability. As a global leader, Karl Storz has set the standard for reusable ureteroscopes. But now, a new contender is entering the spotlight. Jiangsu Mole Medical, a national high-tech enterprise, is challenging the status quo. With a focus on ... Read more

Behind the Scenes with Mole Medical: Inside the World of Leading Video Laryngoscope Manufacturers
In the fast-evolving medical device industry, video laryngoscope manufacturers play a key role in advancing patient care. Mole Medical stands out not just for its products, but for what happens behind the scenes. What makes this company different? Why do so many professionals trust its solutions? One reason is speed. Mole Medical has an R&D ... Read more
How the Medical Video Laryngoscope Operation Enhances Surgical Success
In the heart of a busy hospital, Dr. Emma Liu prepared for one of her most demanding mornings. The emergency room was packed, and a critical patient needed urgent intubation. Years ago, this would have been a stressful and uncertain procedure. But today, Emma felt calm and ready. She trusted the power of the Medical ... Read more



